Commercial aspects
Learn more about the Health Technology Assessment related issues of your ATMP development.
The following resources have been developed through the Swelife-ATMP SDP3 Business Models and Health Economy project.
The goal of the following resources is to increase the likelihood that ATMPs developed or marketed in Sweden will actually be accessible to patients. Risks around commercial considerations during development and at market approval are discussed as well as the importance of healthcare/industry partnerships for implementation and early health economy considerations.
Product developers, healthcare and industry are encouraged to use these resources to increase their understanding of the similarities and differences of ATMP drug development compared to traditional pharmaceuticals and to understand the critical aspects they can contribute to in order to expedite ATMP generation, delivery and reimbursement in Sweden.
Commercial Resources
The final report
The main target group for the report are SMEs. The report acts as a support resource for development of ATMPs highlighting what needs to be done early to make sure products not only make it to market approval but to reimbursement, the best access for patients. As a tool the report investigates Health Economy questions around certain types of ATMPs and proposes solutions that could scale to the National level and to a variety of products.
The report and some of its appendices have been translated from the Swedish versions to English, as this was requested by stake holders during the referral of the report during 2020. Appendices A and B were translated by IHE – The Swedish Institute for Health Economics, the report and appendix D were reviewed and translated by Svensk Språkservice. The translation of the report and appendix D have been edited and corrected in terms of terminology, by members of the project team, into version 3.
Appendix documents to this report;
ATMP Health Technology Assessment checklist (Bilaga A English, Swedish)
Guide to using the ATMP HTA checklist (Bilaga B English, Swedish)
A generic TPP template for ATMP (Bilaga C English)
An explanation of value-based pricing for drug reimbursement (Bilaga D English, Swedish)
Commercial issues regarding regulations in ATMP development (Bilaga E Swedish)
A presentation of Health Economics, Pricing & Reimbursement models for ATMPs in other countries (Bilaga F English)
Initial results of global market analysis of ATMP for China, Japan and South Korea (Bilaga G English)
An introduction to the sakigake fast-track review system (Bilaga H English)
European benchmark analysis of ATMP market entry from an HTA perspective (Bilaga I English)
A high level road map of the pharmaceutical development process for ATMPs (image below – yellow boxes covered in this report) (enlargeable version)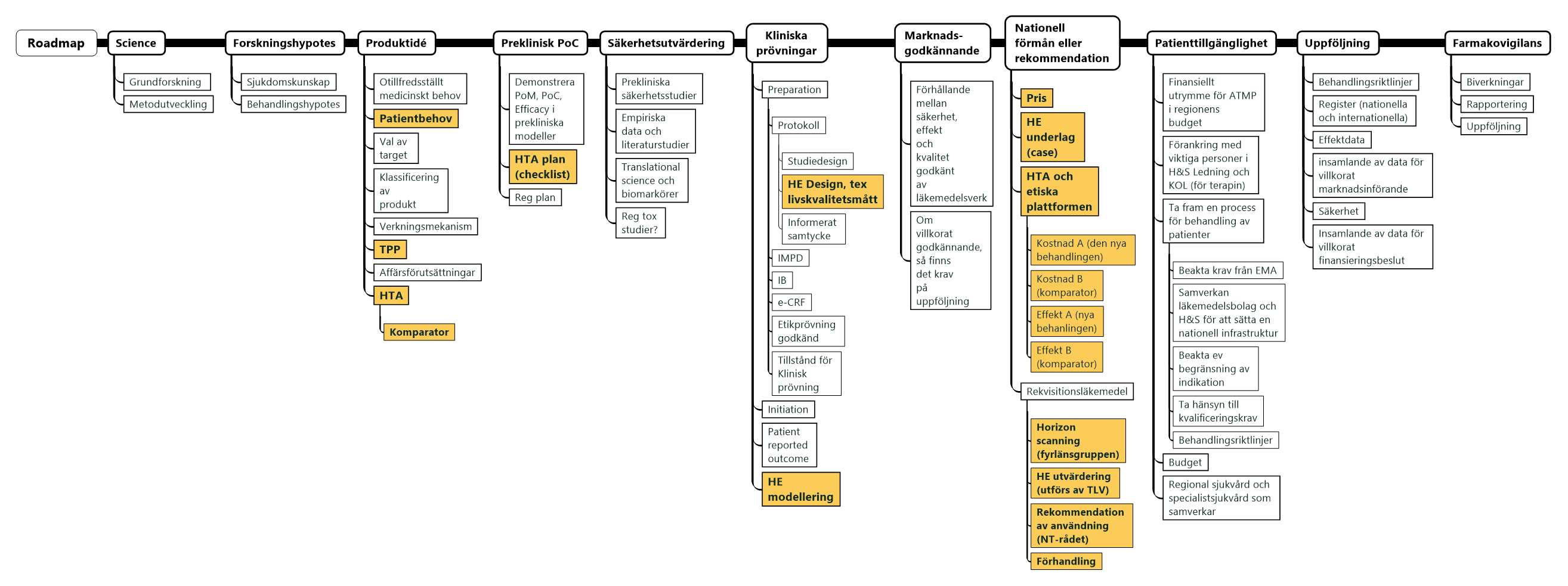
Related content

Find Swedish ATMP activities/facilities and how to engage.

Find or advertise ATMP relevant job vacancies.
