CAMP Projects
Projects within CAMP are operated within three work packages (WP1-3) covering scientific and technological challenges and infrastructure around process development and production of ATMP.
Sixteen collaboration projects engaging partners from healthcare, industry and academy have been, or are currently running within WP1-3.
The current project portfolio consists of the following:
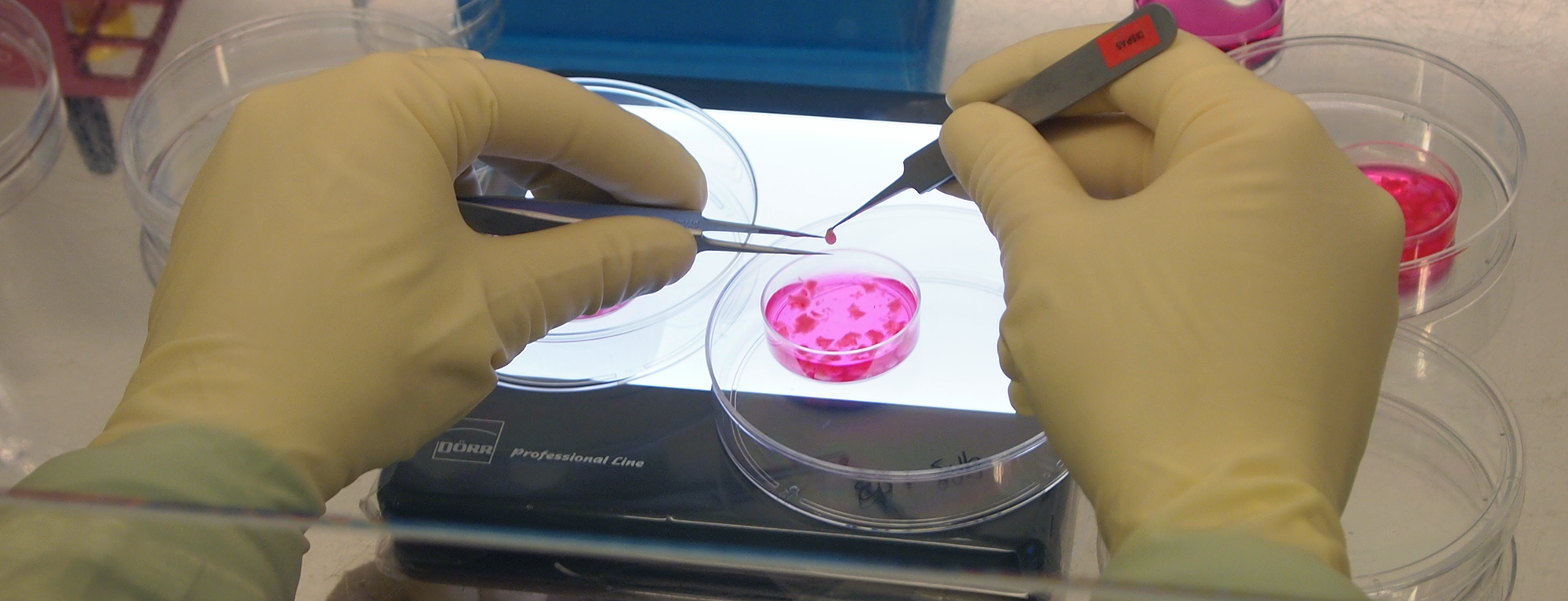
- Development of Multilayered Tissue-Engineered Skin Construct (continuation)
- GMP production of pluripotent stem cells and its derivatives (continuation)
- Extracellular vesicles (CARMEV) for prevention of heart failure (continuation)
- Scaleable magnetic isolation of differentiated stem cells (continuation)
- Personalised ATMP for the regeneration of arteries
- Automated manufacturing of ATMPs
- Pre-GMP development of ATMPs and other gene-based strategies (continuation)
- Subproject 1. GMP education and training courses focused on ATMPs
- Subproject 2. Development of HQ plasmid production (continuation)
- Subproject 3. Development of small-medium scale viral vector production
- Subproject 4. Development of genetically modified T cells (extension of ongoing project)
- Subproject 5. Multi-use RNA production platform for A/GTMPs, and other gene-based interventions
- Vecura/KCC – an infrastructure for manufacturing of ATMPs (continuation)
- Subproject 1. Expansion of GMP Compliant Tumor Infiltrating Lymphocytes (TILs) in Xuri bioreactor – (continuation)
- Subproject 2. Expansion of GMP-compliant adaptive T-cells in bioreactor
- Subproject 3. Cell processing of NK-cells using Sepax cell processing instrument
- GMP-compliant infrastructure and production in a hospital-setting of mesenchymal stromal cells from lipoaspirate for use in clinical trials (continuation)
- Amniotic derived cell therapy improving outcome in lung transplantation – preparations for clinical use
- QC and logistics
For information or with project ideas contact;
Completed projects
- Label-free sorting of stromal cell preparations for clinical use
- ATMP for urethra reconstruction with de- and recellularization technique
- CAR-T cell therapy for malignant melanoma of the skin and the eye
- Value survey – How do donors, patients, the general public and policy makers want to balance different interests?
- Development of producer lines for delivering therapeutic mRNAs through Extracellular Vesicles
- Single cell qPCR characterization of ATMP
- Establishing a non-dry ice dependent logistics strategy for cell therapies
- Establishing a QC and logistics strategy of cell-based products for secure patient treatment.



