2020-12-10

Find information and tools to assist in establishing your business model.
The Swelife-ATMP SDP3 project has now launched their project report! This resource was developed by partners from 14 public and private organisations, to map issues around Business Models and Health Economics for ATMPs and through this expedite ATMP development by conveying greater understanding of processes and critical parameters.
The project was led by Anna Ridderstad Wollberg from the Research Institutes of Sweden (RISE). The rest of the project partners can be found on the project page. You can also find a Swelife interview with Anna on the project here.
The report and associated files can be found in the Commercial aspects of ATMP resource. The final report describes and exemplifies issues around valuation and payment for ATMPs. The importance of developing, not only a product that works scientifically and clinically, but a product with a well thought out business model and a thorough understanding of competing technologies, is critical to navigating not only market approval and commercialisation but also reimbursement in public health care systems. Resources developed in association with the report address those areas shown in yellow in the SDP3 road map (summarised here, high resolution here).
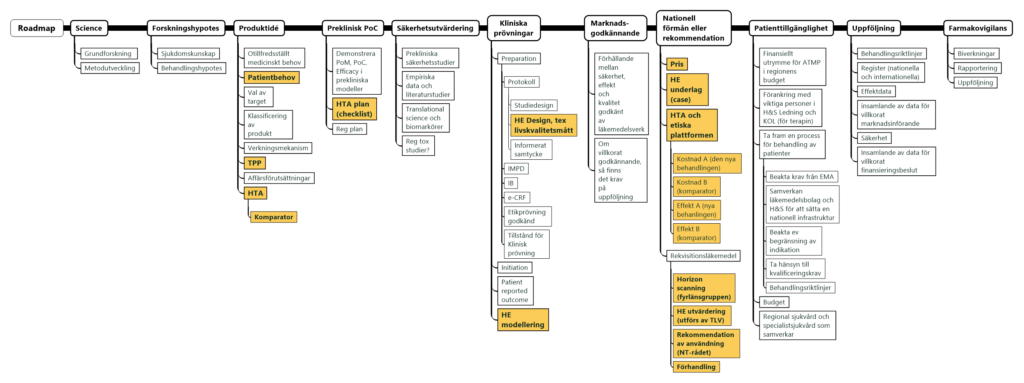
Product developers are encouraged to familiarise themselves with the formal milestones of ATMP development to enable solid planning to reduce financial and development risks, increasing product value. ATMP development follows many of the same principles as ‘classic’ pharmaceutical development and there is a lot to be learned from these established processes/experiences including collection of information proving benefits in relation to risks regarding both efficacy and health economy. The project has developed some very interesting resources, including generic case Target Product Profiles (TPPs) and considerations for early decision-making models on Health Economy valuation and payment, based on these cases. Further, they delve into the prerequisites for development of ATMPs with sustainable business models not only for development and clinical trials but also for market approval and commercialisation.
Healthcare is prompted to prepare for the opportunities ATMP can bring for patients. If healthcare is not able to offer the treatments, even established market approval and reimbursement solutions will not be able to bring the treatment to the patient. For ATMPs this requires new partnerships between public healthcare systems and commercial companies, evidence of the technological advances of this field. These important stake holder interactions and payment models for ATMP are investigated further in work package 3 (WP3) that is spun out from the project and will continue during spring 2021, led by Örjan Norberg Region Västerbotten.
One interesting point is that while the Health Economy assessment of ATMPs is not so different from other novel technologies, the issue of increased uncertainty in comparing long term effects with competitor treatments can be difficult. Design of clinical trials links into this with difficulty to randomise clinical trials and perform studies on a large patient base, due to the nature of the products. Delivering significantly increased survival and/or increased quality of life compared to other available treatments; even life-long single-intervention treatments are opportunities that must be met as cost-effective valuation. However, ATMPs to be delivered for a wide range of conditions, to many people, poses budgetary challenges where financing and payment model structures will need to be developed to ensure patient access.
It is now in Swedish policy through the National Life Science Strategy that Swedish patients are to access effective treatments and that Sweden will be competitive in research product and development.