Welcome to ATMP Sweden!
ATMP Sweden is the national network of Sweden’s activities within medicines based on genes, cells or tissue engineering, classified as Advanced Therapy Medicinal Products (ATMPs) in Europe. Our goal is to promote the collaboration and communication needed for accelerated, effective ATMP based patient solutions.

Find the recordings!
The 27th of June “European Competitiveness in Advanced Therapies: How do we Fulfill the Potential for the Benefit of Patients?”
The 28-30th June ”ATMP world tour 2023″
News

2023-06-06
ARM and ATMP Sweden co-host ’EU competitiveness in ATMP’
Join the 27th of June to discuss potential impact of recent EU legislation changes
Read article
2023-05-24
Welcome to the world CCRM Nordic!
CCRM Nordic AB to utilise 160 million SEK over 2 years to develop ATMP Innovation Cluster
Read article
2023-05-04
ATMP Sweden newsletter April 2023
Brought to you by ATMP Sweden – the national network of Sweden’s activities within Advanced Therapy Medicinal Products (ATMPs)
Read articleWhat are ATMPs?
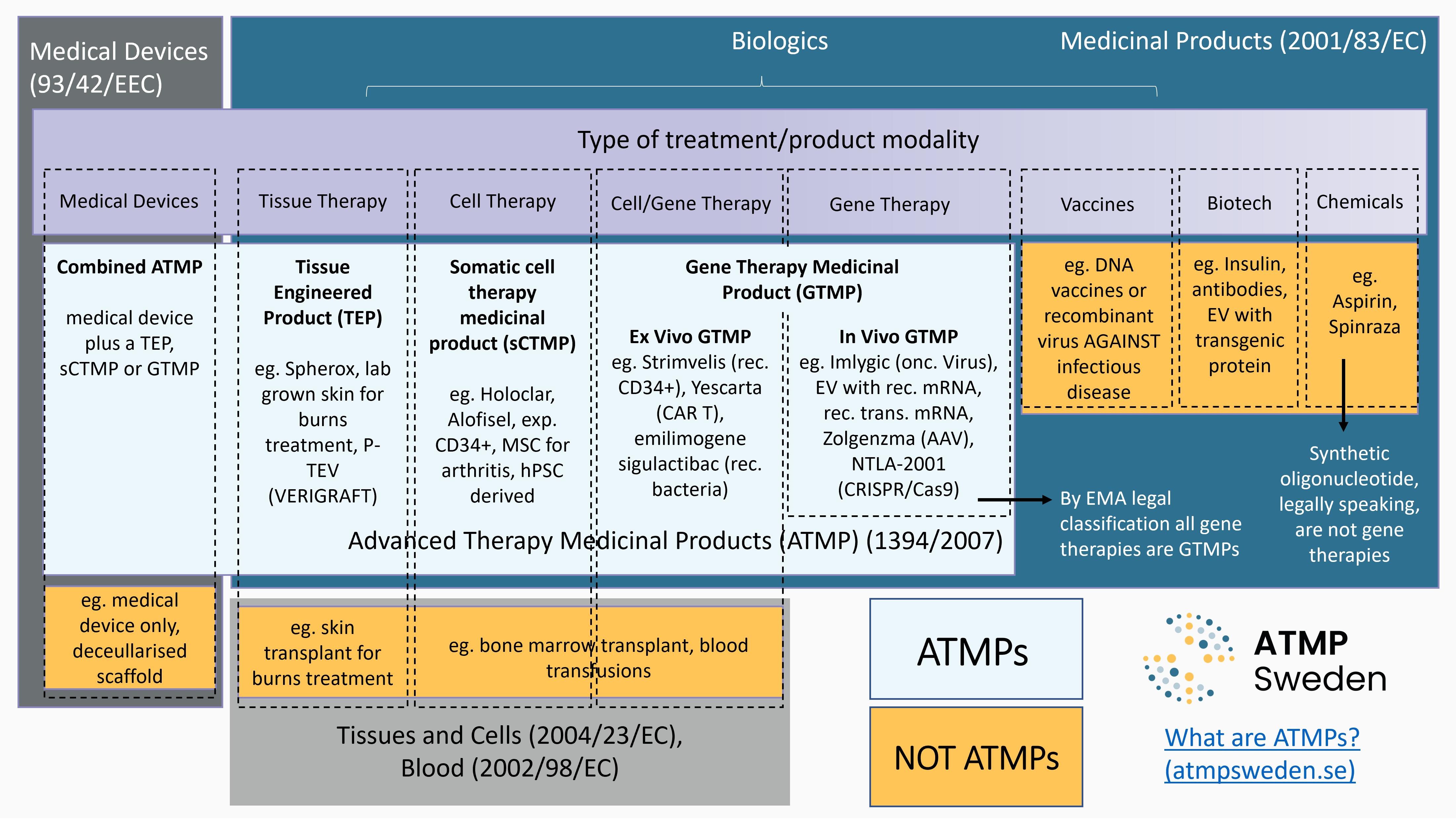
The obvious, less obvious and ATMP related technologies
ATMPs are innovative therapies that encompass gene therapy, somatic cell therapy and tissue-engineered products. With ATMPs, diseases can be treated in completely new ways.
Conferences & events
Resources

Learn more about the Health Technology Assessment related issues of your ATMP development.

This guide will provide useful information and insights throughout the ATMP development process.

Find or advertise ATMP relevant job opportunities.
