ATMP Introductory lectures
The basics of the ATMP field from what are ATMPs to the issues in reimbursing their use.
These lectures are being offered as part of the ATMP Sweden/Karolinska Institutet run “Gene and Cell Therapy Product (ATMP) Drug Development” PhD course.
Registration through ATMP Sweden 2020 -now closed.
PROGRAM ATMP Introductory Lectures – November 16th – 20th, 2020.
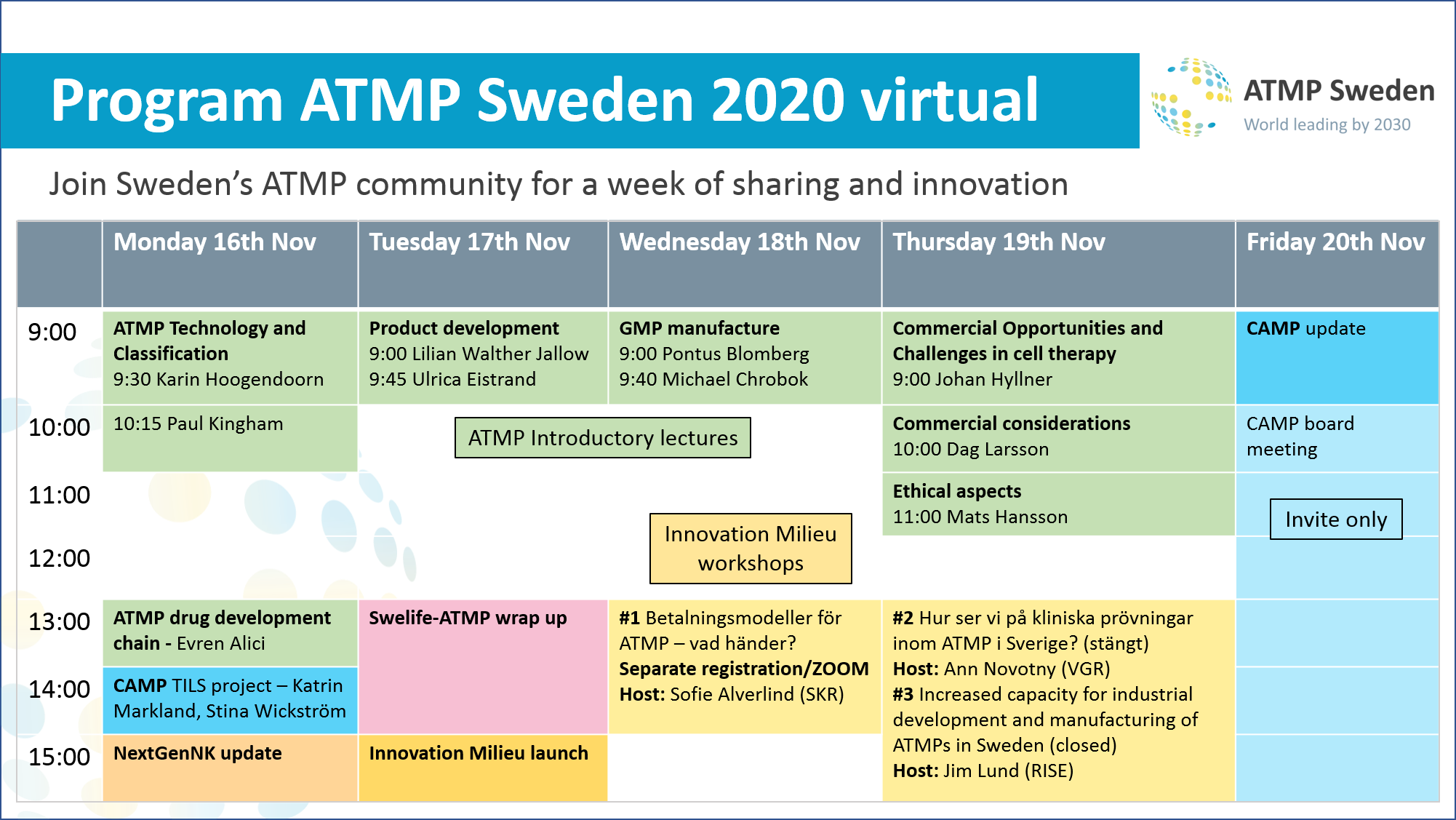
Monday 16th November
ATMP technology and classification
9:30 – 10:15 – What are ATMPs and history – Karin Hoogendoorn presentation on request. But you can find Karin’s previous ”What are ATMPs” presentation from the 17th of June 2020 here.
10:15 – 11:00 – Entering the manufacture environment – Heather Main
presentation slides: Entering the manufacturing environment 161120
The ATMP drug development chain
13:00 – 14:00 – Evren Alici (KI/NextGenNK)
Tuesday 17th November
Manufacturing of ATMP from an academic´s point of view
9:00 – 9:40 – Adaptation of a research product to GMP – Regulatory and quality aspects of manufacturing and planning a future clinical trial. Lilian Walther Jallow, PhD, research coordinator, KI and CEO, BOOST Pharma
presentation slides: phd kurs_manufactuingslides
9:45 – 10.15 – The Manufacturer´s perspective; what to do and what to avoid – Ulrica Eistrand, PhD, Production Manager, Vecura
presentation slides: Requirements for GMP-production process_20201115
Wednesday 18th November
ATMP/GMP manufacture
9:00-9:40 Introduction to ATMPs in Sweden and ATMP manufacturing – Pontus Blomberg (Vecura/KCC, CAMP)
presentation slides: Pontus lecture GMP manufacture 20201118
9:40-10:00 Scale up and scale out – Michael Chrobok (KI pre-GMP)
presentation slides: Scaling up Scaling out Michael Chrobok
Thursday 19th November
Commercial considerations for ATMP
9:00 – 9:45 Opportunities and Challenges in Cell Therapies – Johan Hyllner presentation on request
10:00 – 10:45 Commercial considerations in ATMP – Dag Larsson presentation on request
11:00 – 11:45 Ethical aspects in commercialisation of human tissue – Mats Hansson