2019-09-16

Part one of our summary of ATMP financing events in Skåne September 2019
Tuesday the 10th of September and Wednesday the 11th of September Swedish investors, academic and SME ATMP developers met in Skåne to discuss opportunities and know how within ATMP development financing. The 10th Sept event was held at Medicon Village, Lund, sponsored by Medicon Village, Swelife and Bioneer. Here we summarise some interesting perspectives from the day! Check out part 2 which extends to the panel discussion Sept 11 for later stage products. Please feel free to contact us for more information.

Today’s Medicon Village event showcased financing options within the early stages of ATMP development, from academia to commercialization and/or clinical trial. The event was split between those seeking funding and support and those providing funding and support. Each speaker provided something unique, something to look out for, something to consider.
Compared to classic pharmaceuticals, ATMPs are complex and expensive to produce. While they bring new possibilities to a plethora of currently untreatable diseases the development phase to generating clinical data is longer and more expensive making ATMP products high risk investments. Due to this larger and longer public and philanthropic funding is required to ensure sufficient generation of clinical data to decrease risk, making a therapy more attractive to traditional investment opportunities. Securing and nurturing philanthropic relationships may not only provide critical early development funding but can also create the networks, engagement and trust required for future investments. Establishing benefits to society, why you, why now are key in acquiring grants and philanthropic funds in highly competitive environments. Public funds should be seen as the gateway to investments in ATMP, as a chance to establish viable clinical and commercial strategies….keep in mind that at the 11th September HealthCap investors mentioned how fast and cheap ATMP development is compared to classical pharmaceuticals….an explanation of this comes in part 2 of our finance event summaries.
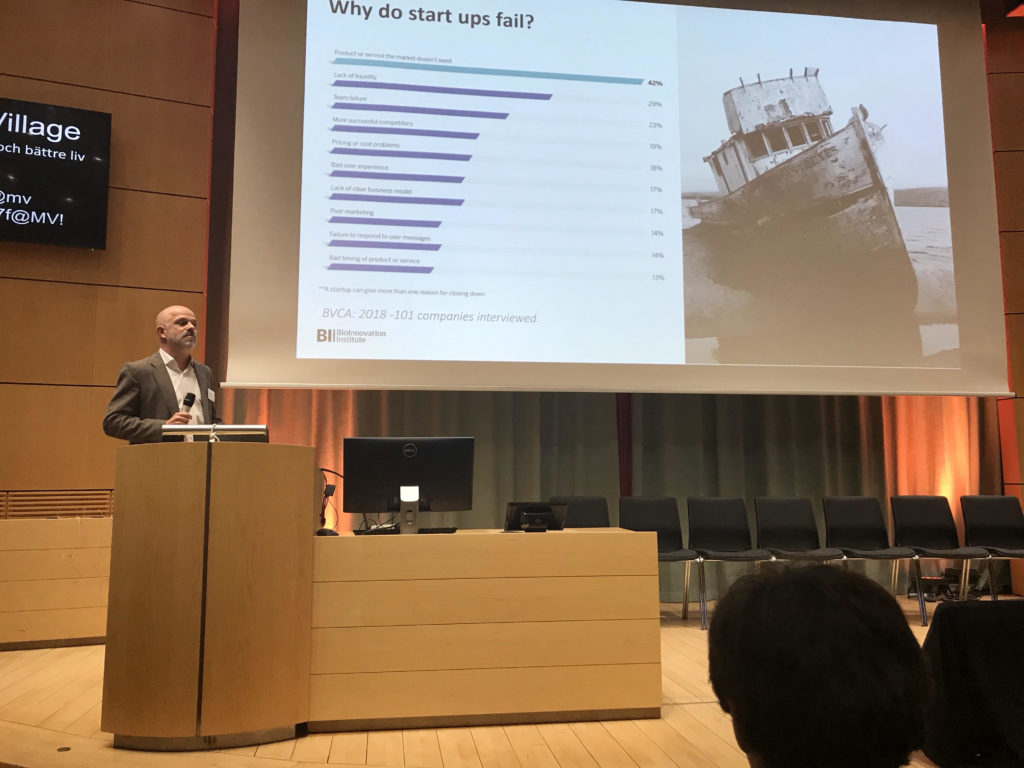 The essential need for early clinical and commercial development within academia can be a problem when the traditional training of academics rarely involves entrepreneurial programs. Thus, close collaboration between academics, healthcare and industry is required to ensure successful generation of ATMP technologies with clear clinical and commercial paths. Whether Swelife, EU, Bio Innovation Institute (BII) or academic incubator programs there are a multitude of support systems available to generate the networks and knowledge needed for the best science to achieve commercial ATMP success.
The essential need for early clinical and commercial development within academia can be a problem when the traditional training of academics rarely involves entrepreneurial programs. Thus, close collaboration between academics, healthcare and industry is required to ensure successful generation of ATMP technologies with clear clinical and commercial paths. Whether Swelife, EU, Bio Innovation Institute (BII) or academic incubator programs there are a multitude of support systems available to generate the networks and knowledge needed for the best science to achieve commercial ATMP success.
Swelife may be best known in the ATMP circles for it’s Swelife-ATMP project; five system development projects, a national conference and communication activities, collectively aimed at identifying needs and developing networks, activities and resources to address these needs. Today we heard of another Swelife project within ATMP, that of Rula Zain and Edvard Smith at Karolinska Institutet. Through the project, funding and access to professional networks is accelerating commercialization of a patent-based, innovative oligonucleotide therapy (OT) for the treatment and prevention of nucleotide triplet-repeat diseases, an area recognized as sustainable innovation by the Swelife team. There are great opportunities within Swelife for establishing ATMP products to prevent and treat debilitating disease.
A team of speakers represented some of the ATMP activities of Novo Nordisk Foundation and Novo Holdings, the holding company of the Novo group. Of particular interest was the Bio Innovation Institute (BII) which provides grant funding and entrepreneurship support to academic stage ATMP projects to ensure suitable clinical and commercial models are established in early development phases. BII is looking for solid science projects run by academics who ‘prefer to remain within academia’. BII’s support is to ensure projects are attractive to the right commercial partners who may then take them forwards. A logical follow-on to BII activities is Novo Seeds, the early stage investment team of Novo Holdings, which can provide seed investments, managerial and strategic support to solid scientific projects. Other teams within Novo Holdings, stageNovo Ventures and Principle Investments, can provide funding for more mature companies. Novo Nordisk Foundation and Novo Holdings are go-to-partners for any phase of ATMP development, from research institutes such as the DanStem Center at Copenhagen University, to startup companies such as Pancryos, and biopharma companies such as Lysogene.
AstraZeneca is active in the ATMP field with at least 2 products under development including stem cell derived Human Ventricular Progenitors in collaboration with Procella therapeutics and VEGF-A modified mRNA in partnership with Moderna therapeutics and Karolinska Institutet. With focus areas in cardiovascular, renal and metabolic diseases, oncology and respiratory, with opportunistic interest in neuroscience, AstraZeneca welcomes contact from academics, industry and scientific organisations towards accessing the best science and complementary technologies. The AstraZeneca website has great tips for approaching partnering contact and runs ‘Open Innovation’ and ‘Externally sponsored research’ programs enabling flexible collaborative potential at various stage of ATMP development.

Asgard Therapeutics, originally supported by the Lund University Innovation team is a great example of an academic spin off run by the technology founders. Asgards innovative gene therapy technology, Trojan DC, aims to revolutionise cancer treatments by reprogramming cancer cells into dendritic cells which will then by design present antigens the cancer has been hiding from the immune system, thus enabling the host immune system to attack the cancer. A fantastic concept is not enough. Funding for reducing commercial risk through Vinnova VFT (verification for growth) as well as assistance with IP, regulatory and financial strategies were essential to moving Asgard through critical phases. Asgard also received an Exploratory pre-seed grant from Novo Nordisk Foundation towards their proof of concept studies currently ongoing.
The final speaker was Cecilia Götherström from EU project BOOSTB4 focusing on severe Osteogenesis Imperfecta (OI). “The network is key”, what began as a VR/KI/KUH initiative expanded to a successful EU project application, which was written in collaboration with a consultancy firm. The application was granted funding allowing initiation of a multicenter clinical trial with partners in 4 European countries, PHARMALEX health economy consultation, MedSciNet clinical trials database and ethical, regulatory and manufacture support from CellProtect Pharmaceuticals and Lund University. A voluntary harmonization procedure (VHP) was a lot of work but refined the clinical trial plan. Wow, what a network! Cecilia does not recommend running clinical trials from an academic setting and recommends involving a CRO from the beginning. She recommends working with an administrative partner that is dependent on your success. Ensure your network is working towards a common goal. Getting the grant is the beginning not the end – it is then that the real challenge starts!
The seminar ended with a panel discussion with several questions from the audience. The discussions continued over coffee and networking with the speakers and the day came to an end with carpooling to the Nordic Life Science Awards and welcome get together at the Nordic Life Science Days in Malmö!
Article written by: Heather Main