Reports
Here you will find links to reports published by our projects.
Find the ’Pilot Study of a Swedish Institute for Cell Therapy’ at the bottom.

Patient- och närstående- samverkan för bättre forskning och hälso- och sjukvård

Lägesrapport och kartläggning av utbildning och framtida kompetensbehov inom ATMP
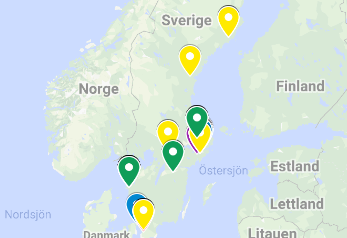
Yearly report of Nordic ATMP status, completed July 2022 by the ATMP2030 National Coordination activity

Read about the Swelife-ATMP initiatives towards identifying ATMP specific gaps in law, ethics and regulation, as well as identifying specific needs within healthcare, business, research and industry.

Infrastructure report completed March 2021 from the ATMP2030 Innovation Milieu Industrial development activity.

Commercial aspects report completed December 2020 by the Swelife-ATMP health economics project.
Utmaningar och möjligheter för stamcells-baserade läkemedel i Sverige och karta över etiskt och legalt ramverk.

Baselining activity within Swedish ATMP completed October 2020.

Regulatoriska aspekter vid ATMP-utveckling

Swelife-ATMP Gene Therapy report completed February 2020 on opportunities and challenges in Swedish gene therapy.
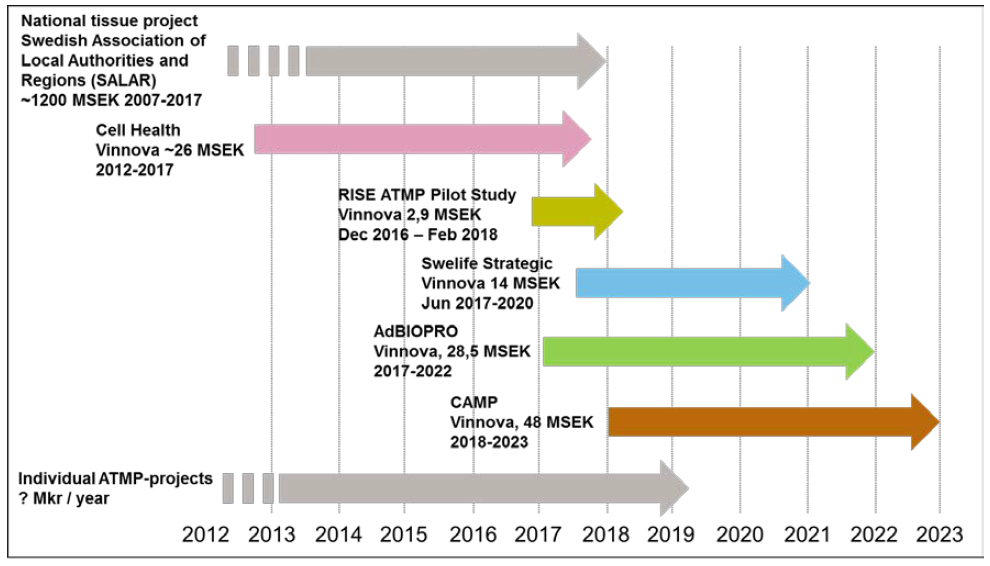
Pilot Study of a Swedish Institute for Cell Therapy published late 2017.
