Tumor Infiltrating Lymphocytes
Expansion of GMP Compliant Tumor Infiltrating Lymphocytes (TILs) in Xuri bioreactor
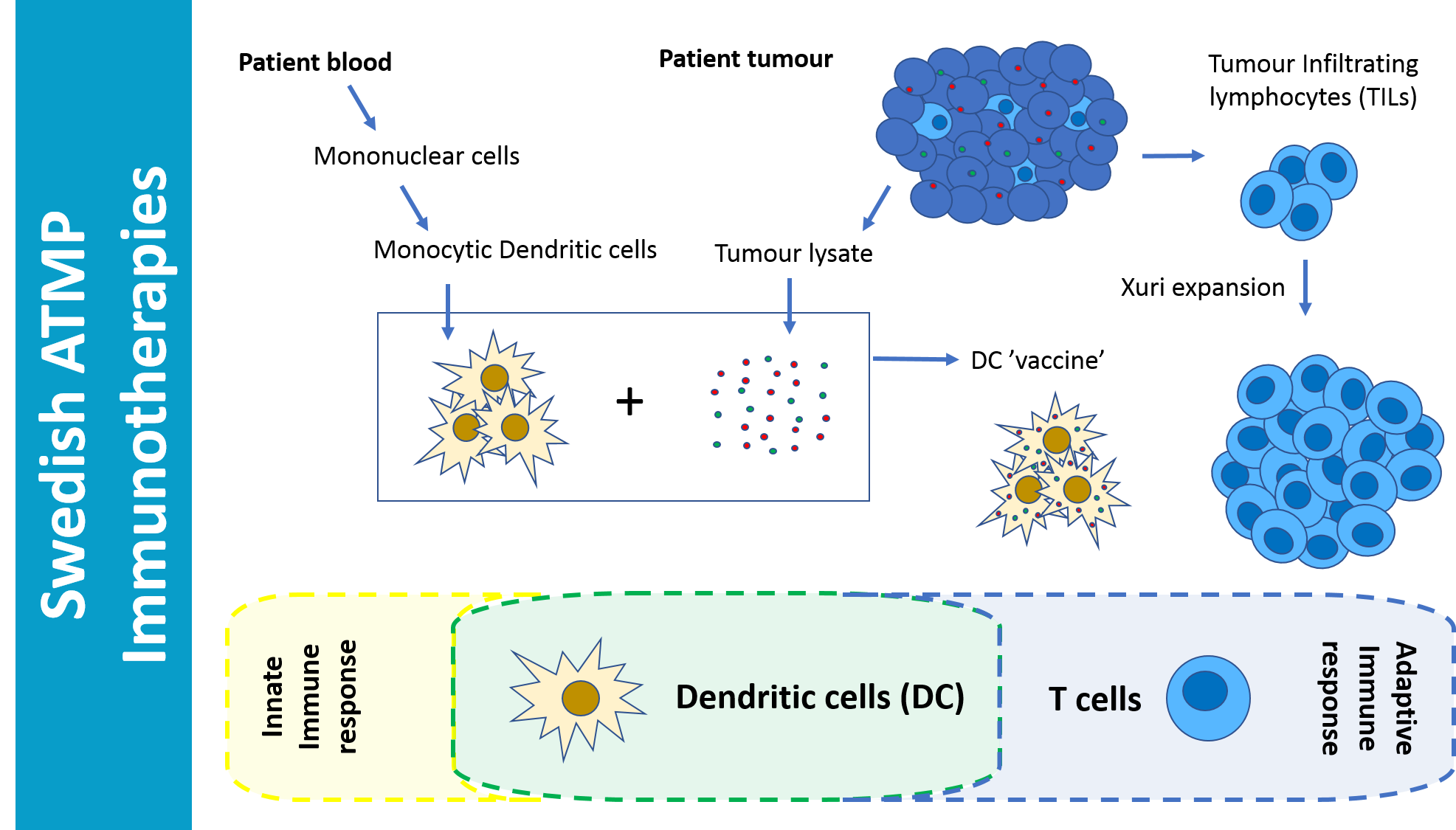
This project is focused on GMP-manufacturing of Tumor Infiltrating Lymphocytes (TILs), using the GE Healthcare Xuri bioreactor, for treatment of malignant melanoma
Time period: 09/2021 – 08/2023
Lead: Pontus Blomberg, Karolinska University Hospital
Involved partners: Cytiva, Karolinska institutet
GMP manufactured TILs are being used together with dendritic cells for the treatment of malignant melanoma. TILs are manufactured using the GE Healthcare Xuri bioreactor. Treatment of the first 10 patients was completed during 2018. An efficient response was observed in the TILs + DC vaccine group. So far, four responders, two of which are now free of tumors and one only has a minimal lesion remaining.
Publications
The following deliverables are identified.
1. Expanded TILs meeting preset release criteria for up to 10 patients
2. Completion of MAT02 clinical trials where products manufactured at the Vecura site have been used
3. Publication describing the clinical outcome
Presentation slides and recording from ATMP Sweden 2020
See News articles referring to this project;
’Double’ cell therapy gives new hope to malignant melanoma patients
Melanoma gone following treatment with TILs and DC vaccination.